Photo – left to right: Fanglei Tong (PhD student), Xize Chen (PhD student), Tony Wang (PhD student), Dr Shanghai Wei (PI, University of Auckland), Professor Wei Gao (University of Auckland), Kirill Panov (PhD student), Associate Professor Peng Cao (University of Auckland), Associate Professor Tilo Söhnel (University of Auckland), Ryan Silk (PhD student)
Advanced alloy anodes for next-generation rechargeable batteries
The project aims to open a novel route for developing better Mg rechargeable batteries by investigating new anodes where Mg is in combination with other metals (alloys) including those with a specific order or structure (intermetallic phases). The Principal Investigator Dr Shanghai Wei works collaboratively with Professor Mark Taylor, Professor Wei Gao, Associate Professor Peng Cao and Associate Prof Tilo Söhnel, leading a team of PhD students at the University of Auckland, to understand the electrochemical charge/discharge behaviour of different Mg alloys with controlled microstructure.
Breaking it down with sustainable resource recovery

Photo: From left – CHEMMAT PhD students Nerischka Thathiah, Susanne Mathews, and Ellen Liew Wymei stand in their Sustainable Resource Recovery lab
Sustainable resource recovery students figure out how to dispose of waste safely and in a way that’s good for the planet
Whey waste is a pain to get rid of. Dairy manufacturers use acid whey to create cream cheese and Greek yoghurt, and sweet whey to create protein powder. But the leftover whey waste neutralises the bacteria that breaks down dairy, which must be broken down by special bacteria in a wet-waste treatment facility to separate the whey into different tanks.
That’s where Masters student Susanne Mathews and the Department of Chemical & Materials Engineering comes in. Mathews is trying to figure out how to hydrothermally treat whey waste so it doesn’t kill the bacteria that breaks down dairy. If successful, the technology can be implemented to discharge whey waste into normal wet-waste treatment facilities without corrupting the disposal process.
‘Tastes like hāngi in a bottle’ – Auckland academics start kānuka business venture

Photo: Auckland University academics start new business venture, making native kānuka into liquid smoke
Two academics are working on a business venture to bring the taste of hāngi to a bottle using native kānuka.
Dr Kiri Dell and Associate Professor Saeid Baroutian from the University of Auckland have used properties from the kānuka tree to create products – including some that can be used in food.
Emerging environmental contaminants of concern in NZ dolphins

Photo: CHEMMAT Lecturer, Dr Shan Yi
In collaborative research led by Professor Karen Stockin at Massey University, University of Auckland academics including Dr Shan Yi investigated the profile of a group of ubiquitous environmental pollutants, known as PFAS, in New Zealand common dolphins. This research is the first PFAS assessment of cetaceans in Australasian waters, contributing essential data to global understandings of PFAS exposure in marine mammals.
You can read more about their research in Stuff and Phys.org.
Fossil tooth fractures and microscopic detail of enamel offer new clues about human diet and evolution
Photo: CHEMMAT Lecturer, Dr Thomas Loho
Dr Thomas Loho co-authored an article in THE CONVERSATION (23 July 2021):
Teeth can tell us a lot about the evolution of prehistoric humans, and our latest study of one of our species’ close relatives may finally resolve a long-standing mystery.
The genus Paranthropus is closely related to ours, Homo, and lived about one to three million years ago. Both Paranthropus and Homo are often considered to have evolved from Australopithecus, represented by the famous fossils Lucy and Mrs Ples.
The Paranthropus group stands out in our family tree because of their massive back teeth, several times the size of ours, and their extremely thick enamel (the outer-most layer of our teeth). This prompted the hypothesis that they ate mostly hard foods, and one of the most complete Paranthropus specimens was dubbed the Nutcracker Man.
Powerful fictions (and some facts): The truth about the harms of EV batteries

Photo: CHEMMAT’s Dr Peng Cao
CHEMMAT’s Associate Professor Peng Cao comments in the below article featured in THE SPINOFF, 8 July:
Fiction: EV batteries aren’t recyclable
BIG proposes collecting a fee when a battery is imported, which would fund the dissemination of batteries for second-life uses or recycling. Dr Peng Cao of the MacDiarmid Institute and the University of Auckland says that EV batteries are completely recyclable – but it’s not profitable and existing methods are polluting. Local recycling options are being explored, and nationwide scrap dealer Metalman hopes to soon offer a recycling service for all common battery types.
The key to controlling landfill emissions

Photo: Landfill gas is created from decaying organic waste. Photo: Getty Images
CHEMMAT’s Associate Professor Saeid Baroutian wrote an opinion piece for Newsroom, titled “The key to controlling landfill emissions” about how to put technology and innovation front and centre if we want to find New Zealand-specific solutions for capturing of landfill gas.
From the UniServices website 28 June 2021
Models of Sustainability
CHEMMAT’s Professor Brent Young
Imagine you’re a factory owner who wants to make your operations more sustainable. Where do you start? If your machines currently run on fossil fuels, they might be expensive to replace or convert. The new equipment might not run smoothly. You might incur unexpected costs. You might need to change your processes. You might even find your carbon footprint doesn’t shrink as much as you expect.
Change is fraught with risk. Risk can be expensive. Yet the rewards of sustainability can be great both for a business’s bottom line and for the planet. That’s why University of Auckland Professor Brent Young is working to help industry de-risk change, particularly in favour of sustainability.
Creating digital twins
To make change more predictable, Prof Brent Young, a member of the Chemical and Materials Engineering department, is working with a number of companies to model process systems by creating digital twins of those systems.
From efficiency to sustainability
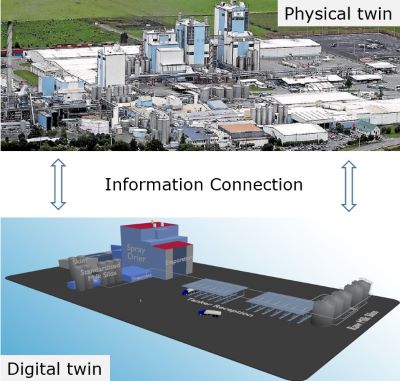
One important use for digital twins is to help industry increase efficiency. Greater efficiency can make processes more sustainable by decreasing energy and material inputs.
Some of the early work Professor Brent Young did in digital modelling was in helping water treatment plants use less energy to achieve the same or better clean-water outcomes. More than a decade ago, he won awards for work he did in that area with the Māngere Wastewater Treatment Plant.
Efficiency is also important when sustainability goals make processes more complex. For example, recycling or repurposing materials that would previously have gone into the waste stream adds additional steps for industry. Digital modelling can help optimise these types of circular-economy processes.
“Efficiency is an important step on the journey to sustainability,” says Young. “However, one thing I’m aware of is the Jevons paradox. William Stanley Jevons was an English economist in the 19th century and he observed that if you make things more efficient, you tend to use them more. If you’re talking about resources, you potentially use more of them rather than less. So from a sustainability point of view, efficiency alone isn’t the answer.”
Modelling new solutions
Digital twins usually start with existing industrial processes. But what if a sustainable new process doesn’t yet exist on an industrial scale? Prof Brent Young is working with colleagues from the University of Auckland Faculty of Engineering to model these too.
With Senior Lecturer Wei Yu, Young is figuring out how best to recover phosphorus from wastewater.
“Phosphorus is in limited supply and there are predictions that if we continue at the current rate, mineable phosphorus might cease to be available within a century, maybe less,” says Young. “However, there’s phosphorus in wastewater, both from fertiliser runoff and from sewage sludge –our urine is one source of it. Technologies do exist to recover that phosphorus for reuse, so we’ve been doing lab work and modelling to understand those processes and optimise them.”
With Associate Professor Saeid Baroutian, Young is working to optimise technologies such as hydrothermal processing and supercritical extraction, which can break down toxic materials and extract valuable compounds from what would otherwise be waste.
From the UniServices website, 14 June 2021
From invasive seaweed to biogas and fertiliser

Photo: CHEMMAT PhD candidate Terrell Thompson and Assoc Prof Saeid Baroutian
Sargassum is a type of algae that normally provides a haven for creatures such as turtles, fish and eels. Since 2011, however, for reasons not entirely understood, unprecedented quantities of sargassum have been invading Atlantic and Caribbean coastlines.
Massive carpets of sargassum as much as a metre thick piled up on Caribbean beaches, driving away tourists. It got tangled in fishing nets and boat motors. It smothered coral reefs and killed sea creatures. One young man named Terrell Thompson saw how sargassum affected his native country of Barbados. He turned to Baroutian for help in solving the problem.
A/P Saeid Baroutian, Thompson’s PhD supervisor, identified two possible solutions. One was using microorganisms. Certain strains of microbes and fungi can digest organic waste and transform it into useful products. Baroutian had been working on this with materials such as food waste and sewage sludge. Seaweed, however, was a challenge because its physical structure and chemical composition made it difficult for the microorganisms to digest.
Enter Baroutian’s second solution. Hydrothermal processing involves breaking down organic waste using water at high temperature and pressure. Baroutian and Thompson found pre-treating sargassum that way breaks it down so microorganisms can almost completely convert it into biogas and biofertilizer.
“Biogas is important for small islands like Barbados because they’re importing energy,” says Baroutian. “Biogas can help replace fossil fuels with a clean energy source. Biofertiliser can help agriculture. The process can also help with wastewater and organic solid waste, since lack of technology and treatment facilities often results in the release of contaminants. So we’re tackling two problems, sargassum and other organic waste, and creating valuable products in the process.”
From waste to win

Photo: CHEMMAT’s Assoc Prof Saeid Baroutian
If traditional Western industry were a diagram, it would be a straight line. Resource extraction leads to production, distribution, consumption and finally disposal. A/P Saied Baroutian is working to bend that line into a circle.
Baroutian, an associate professor of chemical and materials engineering, heads the Waste and Resource Recovery Research Group (WaRe3) at the University of Auckland. He also leads the Master of Engineering Studies programme in Sustainable Resource Recovery, which gets students working closely with industry to solve real-world problems – often leading to jobs for the students.
Fundamentally, Baroutian’s research involves using high-tech processes in the service of principles that would have been familiar to his great-grandparents: minimizing waste, reusing what can be reused and turning things that aren’t useful into things that are.
From waste to high-value compounds
A major problem in the linear economy is that valuable compounds such as metals and minerals end up being disposed of along with low-value waste because they’re too hard to separate. A/P Saeid Baroutian is working to change that.
Separation processes often involve organic solvents such as hexane, acetone and methanol. These solvents, however, are expensive, toxic and flammable, making storing large quantities a health and safety risk.
Unlike organic solvents, supercritical and subcritical fluids are cheap, non-toxic, non-flammable and easily reusable. They’re highly specific, dissolving only the desired compound. They also work much faster than organic solvents.
So, what are supercritical and subcritical fluids and why aren’t they in wider use? To explain, let’s look at a familiar concept. Everyone knows there are three states of matter: solid, liquid and gas, right? However, in-between states can exist.
For example, water boils at 100⁰C and turns into vapour under normal circumstances. But under high pressure, water can remain in liquid form above that temperature. This very hot liquid water is called subcritical, and it’s an excellent solvent.
At temperatures above 374⁰C, water becomes neither liquid nor gas but something with the high diffusion rate of a gas but the ability to dissolve substances like a liquid. This substance is called supercritical, and it too is an efficient and specific solvent at specific temperatures. Carbon dioxide can also be made subcritical and supercritical and used as a solvent.
The challenge is that capital costs are high to start off, because subcritical and supercritical fluids need to be pressurized and pumped. However, the costs can be worthwhile to extract high-value compounds. With mostly renewable electricity used in New Zealand and the ability to keep reusing subcritical and supercritical fluids, it makes for a sustainable solution in the circular economy.
Adding value to an underutilised plant resource
Baroutian is also using supercritical and subcritical water in a project he’s undertaking in collaboration with a Ngāti Porou community of the Whareponga Valley in Ruatōria, East Cape.
The community has a stand of native forest containing plenty of kānuka. Like its better-known cousin mānuka, kānuka is a tree native to Aotearoa New Zealand. A/P Saeid Baroutian has been working with the community to produce high-value products other than honey from the resources it has.
Māori have long used kānuka for its medicinal properties. Baroutian is working with the community to extract bioactive antioxidants and flavours from the leaves using supercritical and subcritical water. The resulting “kānuka juice” is rich in antioxidants, he says.
Another technique, called fast pyrolysis, can produce liquid smoke flavouring. The technique involves burning kānuka wood in the absence of oxygen and collecting the vapours. The lightest vapours become liquid smoke, which has not only a unique flavour profile but strong antibacterial properties.
With the support from Return on Science, an investment committee operated by UniServices, and in partnership with the Māori landowners, Baroutian and University of Auckland Business School lecturer Kiri Dell (Ngāti Porou) have formed a company called Kauruki, which means smoke or haze, to market the liquid smoke.
“It can preserve food because it can completely destroy microorganisms like E. Coli and Listeria,” says Baroutian. “Another advantage is that our product is high in antioxidants and doesn’t contain the toxic compounds that are normally formed when wood is burned. We’ve used it on green shelled mussels and cheeses and the flavours are very nice.”
Greener ways of dealing with waste

hydrothermal technology; plastic, plastic broken down using
hydrothermal technology
Despite all A/P Saeid Baroutian’s research into recovering materials and producing high-value products, there is inevitably some waste that is of no value or that is highly toxic. It’s typically sent to landfills, where it can result in environmental contamination. Baroutian says there’s a better way.
In some countries, hazardous waste is incinerated at high temperatures, but this can result in the formation of toxic compounds such as dioxins and in particulate matter that contributes to smog.
Hydrothermal technology involves breaking down waste with hot, pressurized water and oxygen or nitrogen gas. At high temperatures and pressures, oxygen can be diffused into the water to produce oxidation – that is, burning. However, it occurs at a far lower temperature than in an incinerator, 200 to 300⁰C compared to the 1000⁰C or higher used in hazardous waste incinerators.
“Oxidising breaks down long chain molecules into smaller molecules, so the final products are water, carbon dioxide and maybe some carbon black that can be recovered,” says Baroutian. “Toxic chemicals like those found in pharmaceutical waste, plastics and tyres can be destroyed within 10 minutes into something that is easily discharged.”
Baroutian didn’t invent the processes he’s working with – he’s optimising them. He’s researching catalysts that could further lower the temperatures needed to destroy hazardous waste such as firefighting foam, agrochemical waste and pesticides. He’s working closely with industry to solve their real problems and meet their needs, whether it’s creating mobile units that can dispose of waste on a small scale or designing large-scale facilities.
Baroutian is currently looking for more companies to join him in addressing waste streams as part of a coalition that will minimize risks and costs. Interested parties are invited to contact him at s.baroutian@auckland.ac.nz.
Interested in working with Saeid Baroutian or other University of Auckland researchers in sustainability and cleantech?
Contact Director Strategic Growth Analeise Murahidy at a.murahidy@auckland.ac.nz

